Are you struggling to find the empirical formula in chemistry? Look no further! This article will guide you step-by-step on how to determine the empirical formula with ease.
We’ll explain the concept, provide examples, and highlight common pitfalls to avoid. Understanding the empirical formula is crucial in various real-life scenarios.
So, let’s dive in and unravel the mysteries of empirical formula in chemistry together!
The Importance of Empirical Formula in Chemistry
You should understand the importance of the empirical formula in chemistry. The empirical formula is a representation of the simplest ratio of elements present in a compound. It provides valuable information about the composition and structure of a substance.
By knowing the empirical formula, you can determine the relative proportions of different elements in a compound, which is crucial for various applications in chemistry. For example, the empirical formula helps in predicting chemical reactions, understanding stoichiometry, and calculating the percent composition of a compound.
It also aids in identifying unknown substances and determining their molecular formulas. Moreover, the empirical formula serves as a foundation for further studies in organic and inorganic chemistry, allowing scientists to gain insights into the properties and behavior of different compounds.
Understanding the Concept of Empirical Formula
To understand the concept of empirical formula, you need to grasp the idea of calculating ratios. This involves determining the relative number of atoms in a compound.
Additionally, there are experimental methods that can be used to determine the empirical formula of a substance.
Calculation of Ratios
In order to understand the concept of empirical formula, you need to calculate the ratios between the elements present in a compound. These ratios help determine the simplest whole number ratio of atoms in a compound.
To calculate the ratios, you first need to determine the molar mass of each element in the compound. This can be done by looking up the atomic masses of the elements on the periodic table and multiplying them by the number of atoms of each element present.
Once you have the molar mass of each element, divide each molar mass by the smallest molar mass to obtain the ratio. This ratio represents the number of atoms of each element in the compound and will be used to write the empirical formula.
Experimental Determination Methods
Sometimes, scientists experimentally determine the empirical formula of a compound by analyzing its elemental composition. This method involves various experimental techniques to gather the necessary data.
One common approach is through combustion analysis, where the compound is burned in the presence of excess oxygen to convert all the carbon and hydrogen into carbon dioxide and water, respectively. The amount of carbon dioxide and water produced can then be measured, allowing scientists to calculate the ratio of carbon to hydrogen in the compound.
Another method is elemental analysis, where the compound is subjected to techniques such as mass spectrometry or elemental analysis instruments to determine the amounts of each element present. By comparing these amounts to the molar masses of the elements, scientists can determine the empirical formula of the compound.
These experimental determination methods play a crucial role in understanding the composition of compounds and furthering our knowledge of chemistry.
Step-by-Step Guide to Finding the Empirical Formula
First, you’ll need to determine the number of moles of each element in the compound. To do this, you’ll need the mass of each element and the molar mass of the compound. Start by finding the mass of each element in the compound using a balance or other measuring tool.
Next, divide the mass of each element by its molar mass to find the number of moles. Once you have the number of moles for each element, you’ll need to find the smallest whole number ratio. To do this, divide each number of moles by the smallest number of moles. If necessary, multiply all the ratios by a whole number to get rid of any decimals.
Examples of Finding Empirical Formulas
To begin with, you’ll encounter various examples that demonstrate the process of finding empirical formulas in chemistry. Let’s take the example of glucose, a common sugar molecule.
The molecular formula of glucose is C6H12O6. To find the empirical formula, we need to determine the simplest ratio of atoms in the molecule.
By dividing the subscripts by their greatest common divisor, we find that the empirical formula of glucose is CH2O.
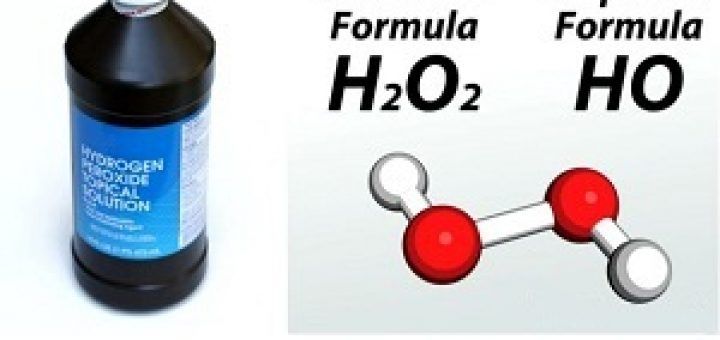
Another example is hydrogen peroxide, with a molecular formula of H2O2. By dividing the subscripts by their greatest common divisor (which is 2 in this case), we find that the empirical formula of hydrogen peroxide is HO.
These examples highlight the importance of simplifying the molecular formulas to their empirical formulas to better understand the composition of compounds.
Common Pitfalls and Challenges in Determining Empirical Formula
When determining the empirical formula, be aware of the common pitfalls and challenges that can arise in the process.
One common pitfall is the presence of impurities in the sample. Impurities can skew the results and make it difficult to determine the true empirical formula.
Another challenge is when the molar mass of the compound isn’t a whole number. In these cases, it can be challenging to find the simplest whole number ratio of atoms in the compound.
Additionally, there may be situations where the empirical formula doesn’t match the molecular formula. This can happen if the compound has a high degree of polymerization or if there are multiple possible arrangements of atoms.
Being aware of these common pitfalls and challenges will help you navigate the process of determining the empirical formula accurately.
Applications of Empirical Formula in Real-life Scenarios
One way you can apply the empirical formula in real-life scenarios is by determining the composition of a compound in a given sample. This can be particularly useful in various industries, such as pharmaceuticals, where the accurate composition of a drug is crucial for its effectiveness.
By analyzing the empirical formula, scientists can identify the elements present in the compound and their respective ratios. This information helps in understanding the compound’s properties and behavior, as well as in developing new drugs or improving existing ones.
Additionally, the empirical formula can also be used in environmental studies to determine the composition of pollutants or contaminants in air, water, or soil samples. This knowledge aids in identifying the sources of pollution and developing strategies for remediation.
Frequently Asked Questions
Can the Empirical Formula Be the Same as the Molecular Formula?
No, the empirical formula cannot be the same as the molecular formula. The empirical formula represents the simplest whole number ratio of atoms in a compound, while the molecular formula gives the actual number of each atom present.
Is It Possible for a Compound to Have More Than One Empirical Formula?
Yes, it is possible for a compound to have more than one empirical formula. The empirical formula represents the simplest whole number ratio of atoms in a compound, so different compounds can have the same ratio.
How Can the Empirical Formula Be Used to Determine the Percentage Composition of a Compound?
To determine the percentage composition of a compound, you can use the empirical formula. It shows the ratio of atoms present, which can be used to calculate the percentage of each element.
Are There Any Limitations to Using the Empirical Formula to Represent a Compound?
There are limitations to using the empirical formula to represent a compound. It only gives the simplest whole number ratio of elements and does not provide information about the actual arrangement or structure of the atoms.
Can the Empirical Formula of a Compound Change Under Different Conditions or Reactions?
The empirical formula of a compound can change under different conditions or reactions. It may vary depending on the ratios of elements involved, altering the composition and resulting in a different empirical formula.
Conclusion
In conclusion, understanding the empirical formula is crucial in chemistry as it provides valuable information about the composition of a compound.
By following a step-by-step guide, one can easily determine the empirical formula of a substance.
However, it’s important to be aware of common pitfalls and challenges that may arise during the process.
Overall, the empirical formula has practical applications in real-life scenarios, making it an essential concept to grasp in the field of chemistry.